How Do Fats Oils and Waxes Interact With Water
In a fat molecule the fatty acids attach to each of the glycerol molecules three carbons with an ester bond through an oxygen atom Figure. Accordingly we provide you with all hints and cheats and needed answers to accomplish the required crossword and find a final word of the puzzle group.

Lipids Fats Oils Steroids And Waxes Lipid Basics Lipids Are Made Mostly From Carbon And Hydrogen They Are Hydrophobic And Don T Dissolve In Water Ppt Download
Low solubility in water.
. Liquid water has fewer hydrogen bonds than ice Oils and fats not have any polar part and so for them to dissolve in water they would have to break some of water s hydrogen bonds. Water will not do this so the oil is forced to stay separate from the water. Some examples of nonpolar molecules include fats oils and waxes.
However despite both being viscous and water insoluble chemicals there are differences between wax and oil that will be highlighted in this article. CodyCross Fats waxes and oils insoluble in water Answers. In dry rendering the animal material is heated usually to 115C135C while being agitated to prevent charring thus releasing its fats oils and waxes as it cooks in its own moisture.
The reason this happens is because of the chemical nature of oil and water. Therefore they do not interact with each other and will separate. In a normal man weighing 70 kg at least 10-20 of the body weight is lipid the bulk of which is triacylglycerol TAG.
Chemicals that dont mix are said to be immiscible. Some examples of nonpolar molecules include fats oils and waxes. Some examples of non polar molecules includes fats oils and waxes.
Some examples of nonpolar molecules include fats oils and waxes. Joining three fatty acids to a glycerol backbone in a dehydration reaction forms triacylglycerol. Polar and nonpolar do not mix.
How do these substances interact with water. They separate from the water. They are described as hydrophobic or water fearing.
Sodium and chloride ions are attracted to charged regions on molecules of polar solvents such as water. You may have experienced examples of how oil and water dont mix. Three water molecules release in the process.
Most fats and oils upon hydrolysis yield several fatty acids as well as glycerol. Hydrilla hydrilla verticillata is an invasive aquatic plant and one of the most serious aquatic pests in florida. Waxes steroids and fats are all lipids which are essential for living cells.
What prediction would you make about why oil and water interact in the way described above. The phospholipid bilayer is the major component of all cellular membranes. In contrast margarine is a wo emulsion containing droplets of water or skim.
How do these substances interact with water. Diagram of the steroid testosterone. Oil and vinegar salad dressing separate.
Fats and oils are nonpolar so they will remain separate from molecules of a polar solvent such as water. The lipids all have a low solubility in water and their characteristics are impacted by the types of bonds that form between. Both wax and oil are sticky products.
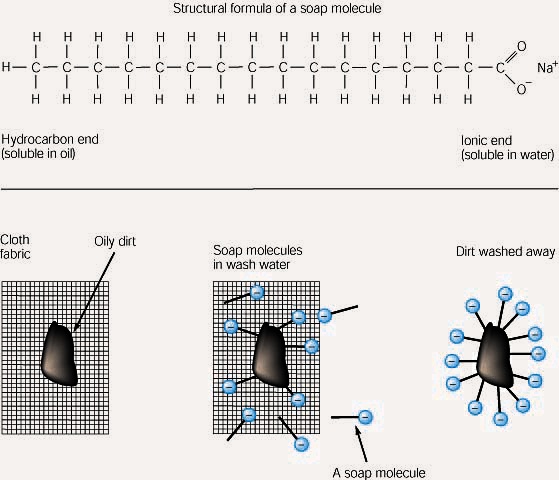
The fatty acid chains are hydrophobic and cannot interact with water whereas the phosphate-containing group is hydrophilic and interacts with water Figure 9. Soap cleans oil and grease because one end of the soap molecule is polar and. Waxes function to provide a waterproof coating on a surface.
Water is charged positively on the oxygen end and negatively on the hydrogen end. How do fatsoilsand waxes interact with water - 1756751 Brianaolmos Brianaolmos 09142016 Biology High School How do fatsoilsand waxes interact with water 1 See answer Brianaolmos is waiting for your help. How do these substances interact with water They separate from it there arent any electrons left over for them to bond with so they stay separate.
Some examples of nonpolar molecules include fats oils and waxes. In fact triglycerides can store much more energy than carbohydrates because they contain so many more bonds. Up to 24 cash back 16.
Motor oil floats on top of the water in a puddle or in an oil spill. This is why fats contain more calories a measure of energy than sugars do. They occur in membranes.
Hydrilla has already been introduced to hundreds of bodies of water throughout florida hydrilla is. Nonpolar molecules do not dissolve easily in water. 2 Show answers Another question on Biology.
Wax is a semisolid substance at room temperature that melts to become a low viscosity liquid at around 45 degrees Celsius. Structure of fats and oils. The bonding in electrons in the molecules are equally shared.
All fats and oils are naturally occurring esters formed from condensation reactions between the alcohol glycerol and. Most animal fats such as those from meat milk and eggs are relatively rich in saturated fatty acids but contain a rather low content of. What prediction would you make about why oil and water interact.
Liquid water is held together by hydrogen bonds. Simple emulsions are either oil suspended in an aqueous phase ow or water suspended in oil wo. The fats oils and waxes sink to the bottom of the vat and can be run off.
What do fats oil and waxes interact with water. How do these substances interact with water. See answer 1 They dont because they dont both have charges.
What arrangement of electrons would result in a nonpolar molecule. Add your answer and earn points. In fact this topic is meant to untwist the answers of CodyCross Fats waxes and oils insoluble in water.
These molecules are referred to as nonpolar. Some molecules that are covalently bonded do not have a difference in charge across the molecule. Low solubility in water.
Po Hydrogen bonds 17. How emulsions and emulsifiers work. How do these substances interact with water.
Milk is an example of an ow emulsion in which the fat phase or cream forms tiny droplets within the skim milk or water phase. Oil is nonpolar and water is polar. No matter how much you mix oil and water they always separate.
Because they are hydrophobic they can form a coating that repels water. The correct answer is B.

Analysis Of Fats Oils Amp Waxes

Lipids Fats Oils Steroids And Waxes Lipid Basics Lipids Are Made Mostly From Carbon And Hydrogen They Are Hydrophobic And Don T Dissolve In Water Ppt Download

Concept 5 3 Lipids Include Fats And Steroids Lipids Group Of Organic Compounds That Include Fats Oils And Waxes Composed Of Carbon Hydrogen And Ppt Download
Comments
Post a Comment